Choose the Correct Compound for the Given Ir Spectrum
Correct cm 1 H 1 The absorbance at 2350 suggests a triple bond is present. The percentage of other gases in dry air is _ The percentage of other gases in dry air is _ Q.
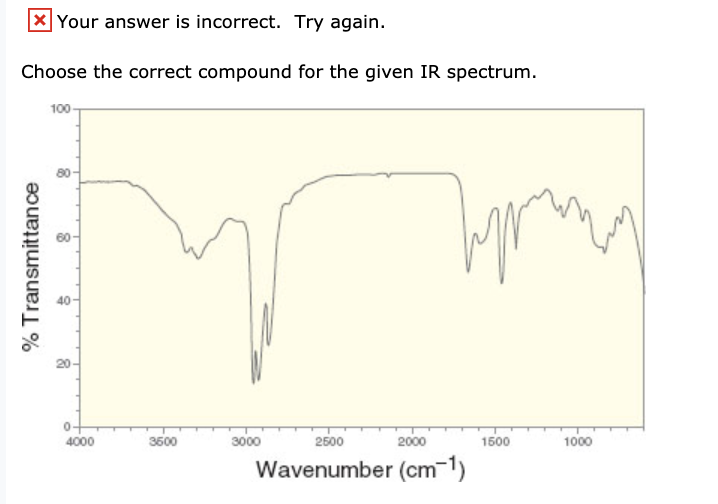
Solved Your Answer Is Incorrect Try Again Choose The Chegg Com
1Circle the letter of each sentence that is true about Daltons Atomic Theory aAll elements are composed of tiny indiv.
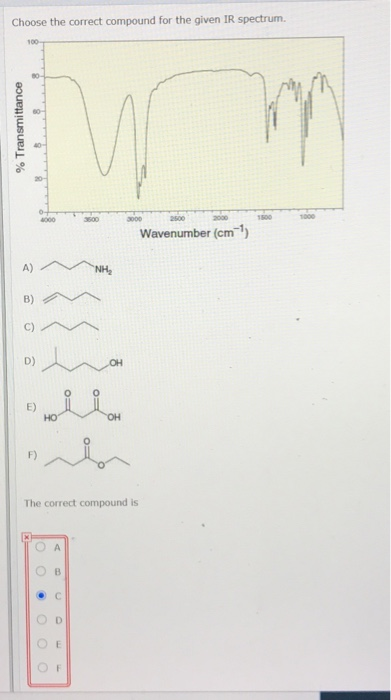
. We review their content and use your feedback to keep the quality high. It works by shining infrared light through the organic compound we want to. Thus in the region above 1500 cm-1 in the IR spectrum of propanone there are two bands corresponding to the C-H stretch and the CO stretch.
Visible light is just a portion of the electromagnetic spectrum and its the infrared section of the spectrum thats utilised in this technique. 100 80 Transmittance Hyry 40 20 4000 3600 3000 2500 2000 1600. Note that not all frequencies have a related compound.
Because if we look into the bands around 2900-3000 cm-1 which will find some traces of the C-H bonds inside the given data. Which one of the following compounds is consistent with the following IR spectrum. With CO peak at 1687cm-1 CO in conjugation aliphatic C-H and aromaticvinyl C-H.
9 10 11 12 13 14 15 16 25 100 4 45 55 6 80 b 60 40 20 4000 3500 3000 2500 2000 1800. Question 7 Part A One of the following compounds is responsible for the IR spectrum shown. Which compound would be expected to show intense IR absorption at 1746 cm-1.
F The correct compound is x. While the CO intense bands are found in a very low level inside. D None of the above.
V FREE Expert Solution Show answer Answer. The only compound with a triple bond in the list is phenylacetonitrile. 1 5 hexadiene.
Choose one of the following. For the three infrared spectra below pick out the molecule from the list that would correspond to the spectrum for that compound. To use an IR spectrum table first find the frequency or compound in the first column depending on which type of chart you are using.
Label the functional groups and identify the correct compound based on the IR spectrum. Start your trial now. Infrared spectroscopy is a particular technique that can be used to help identify organic carbon-based compounds.
OH The spectrum belongs to compound select. One of the following compounds is responsible for the IR spectrum shown. Choose the correct compound for the given IR spectrum.
Wavelength mm 35 7 8. First week only 499. Upon the analyses of the compounds characteristics or properties we get to know that the compound is the one responsible for generating signals inside the IR spectrum as shown.
Choose the compound that matches the IR spectrum shown. The value for absorption is usually in cm -1. Identify how IR spectroscopy might be used to monitor the progress of the following reaction.
AIEEE Bank Exams CAT. 100 7 ratings There is N-H stretching peak one sp3 C-H stretching pea. Then find the corresponding values for absorption appearance and other attributes.
Choose the structure of the responsible compound. Determine what kinds of bondsfunctional group are present and select presentabsent. The vinyl bending and other stretches were matched with the above structure.
Alcohol Secondary Amine O-H N-H Present OR Absent Carboxylic Acid O-H Present OR Absent Primary Amine -NH2 Present OR Absent. It is used by chemists to determine functional groups in molecules. The region below 1500 cm-1 is called the fingerprint region and is characteristic of the molecule as a whole.
While the aldehyde CO stretch is sharp and well-defined the carboxylic CO stretch is broader and more smeared. 100 404 ratings Sign up for free to keep watching this solution. 100 Kw 7 50 68 4000 3500 3000 2500 2000 1500 1000 Wavenumber cm-I 500 HO A.
While the alcohol OH stretch is broader the carboxylic OH stretch is less broad. Solution for Identify the compound from the IR spectra. View the full answer.
C 1 5 Hexadiene. With O-H stretch at 3414 cm-1 aliphatic C-H and aromaticvinyl C-H below and above 3000 cm-1 must be. Identify how IR spectroscopy might be used to monitor the progress of the following reaction он O 3200-3600 As the reaction proceeds the IR spectrum will show signals appearing at cm and signals.
See Spectrum Image Attached. Weve got the study and writing resources you need for your assignments. The main differences between these molecules IR spectra are in the OH stretches and in the CO stretches.
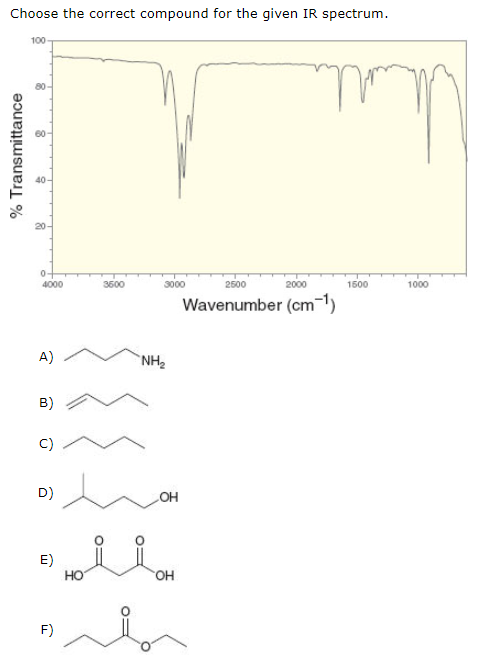
Experts are tested by Chegg as specialists in their subject area. Choose the correct compound for the given IR spectrum 100 Transmittance 000 Wavenumber cm A ΝΗ B C D H E ОН F The correct compound is x. Infrared spectroscopy involves the interaction of infrared radiation with matter.
B Trans 4 Octene. The infra red spectrum can be used to determine the bonds present in a molecule. The sex attractant of the codling moth gives an IR spectrum with a broad signal between 3200 and 3600 cm¹ and two signals between 1600 and 1700 cm¹.
Hence the c c stretch vinyl bending and stretch were matched with the above structure. Look at the given IR spectra for compound Z. In the following IR practice problems we will identify the compound consistent with the IR absorption peaks following three simple steps.
5 items choose the correct answer brief solution is appreciated more 1. In the mass spectrum of this compound the molecular ion peak appears at mz 196 and the relative abundances of the molecular ion and the M1 peak are 272 and 39 respectively. C 1 5 Hexadiene.
Therefore the given spectrum belongs to 1 5 hexadiene. Click in the answer box to display choices. Choose the structure of the responsible compound.
O 3200-3600 As the reaction proceeds the IR spectrum will show signals appearing at cm and signals disappearing at just below 3000 cm.
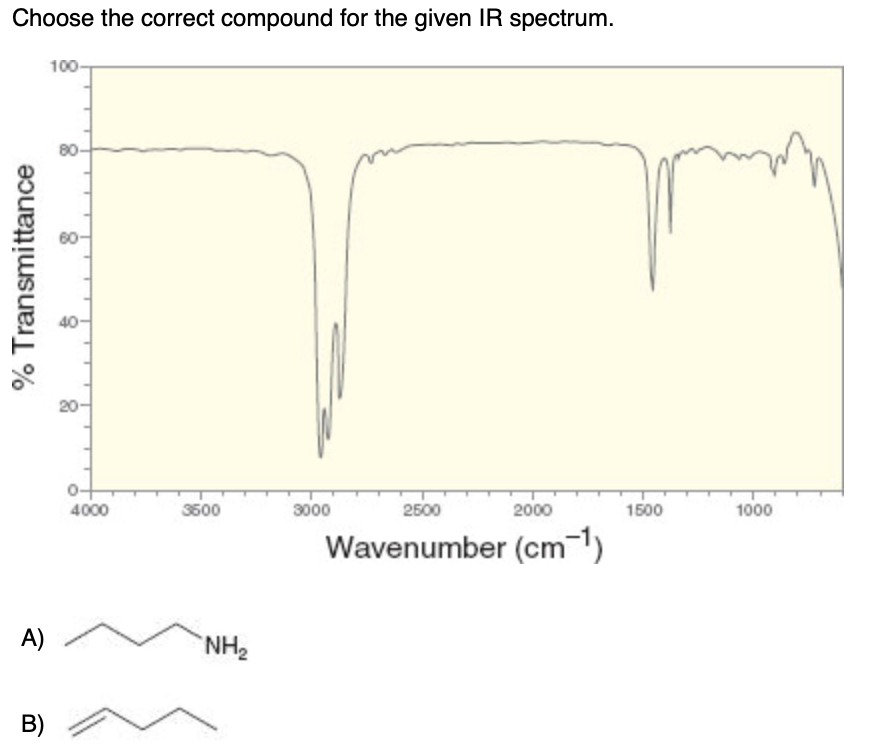
Solved Choose The Correct Compound For The Given Ir Spectrum Chegg Com
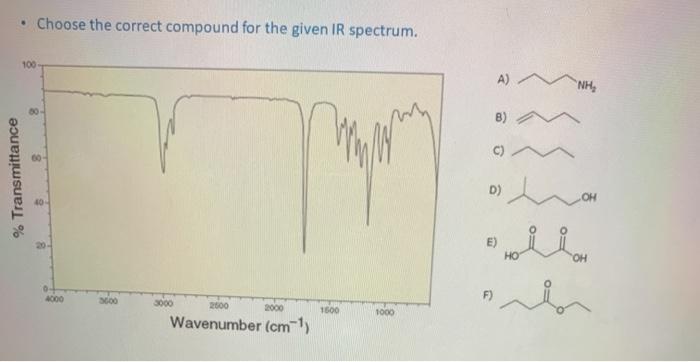
Solved Choose The Correct Compound For The Given Ir Chegg Com
Solved Choose The Correct Compound For The Given Ir Chegg Com

Solved Choose The Correct Compound For The Given Ir Spectrum Chegg Com
No comments for "Choose the Correct Compound for the Given Ir Spectrum"
Post a Comment